Is Rapid Clear Clean Pee Lab Grade
American Cleanroom Systems is a total-service manufacturer, specializing in the rapid production and installation of superior quality custom Pharmaceutical, Medical and Industrial cleanrooms.
Our squad can blueprint, manufacture and install your certified cleanroom in as little every bit four weeks, on-site with minimum disruption.
View FAQs most Cleanroom Classifications
United states of america FED STD 209E Cleanroom Standards | Cleanroom Classifications
| Grade | Maximum Particles/ft³ | ISO equivalent | ||||
| >0.one um | >0.two um | >0.3 um | >0.5 um | >5 um | ||
| 1 | 35 | 7 | 3 | 1 | ISO3 | |
| 10 | 350 | 75 | xxx | x | ISO4 | |
| 100 | 100 | ISO5 | ||||
| 1000 | 1000 | 7 | ISO6 | |||
| 10,000 | 10,000 | 70 | ISO7 | |||
| 100,000 | 100,000 | 700 | ISO8 | |||
ISO 14644-1 Cleanroom Standards | Cleanroom Classifications
| Class | Maximum Particles/m³ | FED STD 209E equivalent | |||||
| >0.1 um | >0.2 um | >0.3 um | >0.five um | >1 um | >5 um | ||
| ISO 1 | ten | 2 | |||||
| ISO 2 | 100 | 24 | x | four | |||
| ISO iii | 1,000 | 237 | 102 | 35 | 8 | Class i | |
| ISO iv | 10,000 | ii,370 | 1,020 | 352 | 83 | Class 10 | |
| ISO 5 | 100,000 | 23,700 | x,200 | three,520 | 832 | 29 | Class 100 |
| ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
| ISO 7 | 352,000 | 83,200 | two,930 | Class 10,000 | |||
| ISO eight | 3,520,000 | 832,000 | 29,300 | Class 100,000 | |||
| ISO 9 | 35,200,000 | 8,320,000 | 293,000 | Room Air | |||
Design Requirements for Cleanroom Classifications
| Criteria | Class 10 ISO4 | Class 100 ISO5 | Class 1000 ISO6 | Class 10,000 ISO7 | Grade 100,000 ISO8 |
| Air changes per HR/Min | 500-600 / 8 to 10 | 300 to 480 / 5 to 8 | 180 / 3 | 60 /i | 20 /0.33 |
| Filter coverage % | 90 – 100 | 60 – 70 | 20 – 30 | seven – 15 | 4 – 5 |
| CFM per square foot | 85 – xc | 36 – 65 | 18 – 32 | 9 – sixteen | 4 – 8 |
| Filter Efficiency | 99.9997% ULPAs | 99.997% HEPAs | 99.997% HEPAs | 99.997% HEPAs | 99.97% HEPAs |
| Ceiling Type | Aluminum T-bar grid | Aluminum T-bar grid | Aluminum T-bar filigree | Conventional T-bar grid | Conventional T-bar grid |
| Low-cal Fixture type | Tear drop or Flow thru | Tear drop or 2'x4' cleanroom fixture | 2'x4' cleanroom fixture | two'x4' cleanroom fixture | 2'x4' standard fixture |
| Ceiling Panel | FRP, Vinyl rock or Mylar | FRP, Vinyl rock or Mylar | Vinyl stone or Mylar | Vinyl stone or Mylar | Vinyl rock or Mylar |
| Wall System | Modular or standard congenital | Modular or standard congenital | Modular or standard built | Modular or drywall | Modular or drywall |
| Flooring comprehend | Welded canvas vinyl or Epoxy | Welded sheet vinyl or Epoxy | Welded Sail vinyl or Epoxy | Sheet vinyl or VCT | Sail vinyl or VCT |
| Flooring base | ii" to 6" cove | Cove or Aluminum base aqueduct | Cove or Aluminum base aqueduct | Cove or Aluminum base of operations aqueduct | Cove or Aluminum base aqueduct |
| Air Returns | Raised floor or center returns | Low wall on long axis | Depression wall at perimeter | Low wall | Depression wall or ceiling |
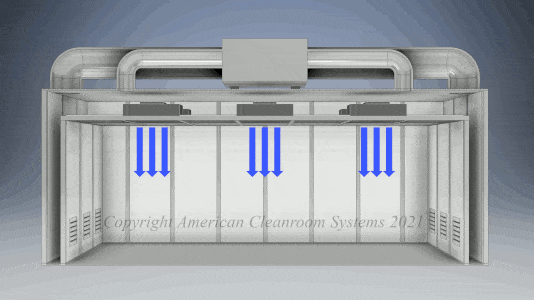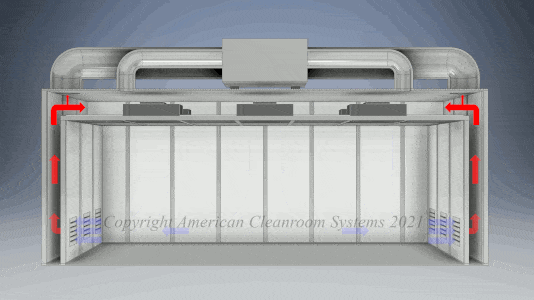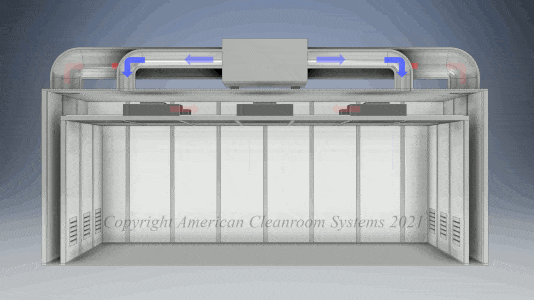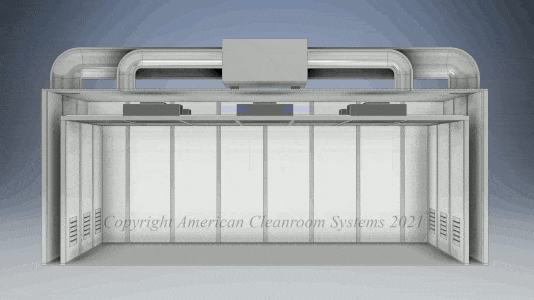
Cleanroom Types:

Modular Cleanroom
A Modular Cleanroom is a cleanroom built using prefabricated modular components then assembled on-site. Why choose modular cleanrooms?
- Modular cleanrooms can be manufactured and installed fifty% faster than stick built conventional construction cleanrooms.
- Unlike conventional construction cleanrooms, modular cleanrooms are easily modified, expanded, or relocated.
- Modular cleanrooms can be congenital for whatsoever classification (class 100-100k, ISO5-ISO8) with temperature and RH control.

Hybrid Cleanroom
A Hybrid Cleanroom is a cleanroom congenital combining modular components with existing conventional construction walls. When to employ a hybrid cleanroom design?
- Combine modular cleanroom air returns and internal walls with existing conventional structure walls can offer toll savings.
- The use of modular cleanroom components significantly speeds up installation of cleanroom.
- Hybrid works best for less enervating ISO-7 or ISO-viii form cleanrooms.

Softwall Cleanroom
A Softwall Cleanroom is a cleanroom created by hanging articulate vinyl curtains on an aluminum frame to create walls.
- Softwall cleanrooms are i pass. Filtered air is pulled by HEPA fan filter units into the cleanroom. The air passes under the curtain back into the surrounding room.
- Softwall cleanrooms tin be mounted on casters so they can be hands relocated.
- Unlike recirculating modular cleanrooms, you cannot add ac to softwall cleanrooms.

Medical Device Cleanroom
A Medical Device Cleanroom is a cleanroom that is used to manufacture medical devices.
- Medical device cleanrooms are designed to provide a controlled surround as specified past the device approved FDA validation and CGMP practise.
- The modular cleanroom is optimized to create a sterile manufacturing environment for the medical devices.
- Often FRP modular cleanroom walls are chosen for medical device cleanrooms considering of the frequent cleaning done in these types of cleanrooms.

Pharmaceutical Cleanroom
A Pharmaceutical Cleanroom is a cleanroom that is used for pharmaceutical manufacturing.
- Pharmaceutical cleanrooms are designed to provide a controlled environment equally specified by your approved FDA validation and CGMP do.
- The modular cleanroom is optimized to create a sterile manufacturing environment for the pharmaceutical products.
- Filling rooms are typically ISO5 class 100.
- FRP modular cleanroom walls are standard due to the ambitious chemicals used to clean pharmaceutical cleanrooms.

Turnkey Complete Cleanroom
A Turnkey Consummate Cleanroom is when the modular cleanroom company provides beginning to end service, doing all aspects of the cleanroom project. This includes:
- Modular cleanroom design
- Manufacturing of modular cleanroom material
- Installation of modular cleanroom
- HVAC, electrical, and flooring provided
- Certification of modular cleanroom

Mask Manufacturing Cleanroom
A Mask Manufacturing Cleanroom is a cleanroom that is used for manufacturing of K95 and surgical masks during the Covid-19 crisis.
- The cleanroom is designed to provide a sterile controlled environment due to mask nomenclature as medical device
- Special modular cleanroom blueprint for rapid manufacturing in mill and installation past customer in field
- Using modular cleanroom walls systems allowed existing warehouse to exist quickly converted into cleanroom at reasonable cost

Light amplification by stimulated emission of radiation Cleanroom
A Laser Cleanroom is a cleanroom optimized for sensitive laser experiments.
- Cleanroom blackout curtains
- HEPA filtration to remove air particulates in cleanroom that could influence experiments
- Tight temperature and humidity control for the cleanroom space

Static Dissipative Cleanroom
A Static Dissipative Cleanroom is designed to prevent static from building up inside the cleanroom which tin damage sensitive electronic components. Features include:
- Static dissipative modular cleanroom walls
- Static dissipative cleanroom floor
- Humidity control
- Ionizer bars

E-liquid Cleanroom
An Eastward-liquid Cleanroom is designed for mixing of East-liquids for electronic cigarettes.
- Due east-liquid cleanrooms provide a controlled make clean environment for manufacturing of due east-liquid ingredients per government regulations
- Modular cleanroom features issue in quick installation and like shooting fish in a barrel expansion of the eastward-liquid cleanroom
- Modular cleanroom is an economical solution for a smaller size cleanroom used by many eastward-liquid companies.

USP797/800 Cleanrooms
USP797/800 Cleanrooms are used for compounding pharmacies.
- Sterile or negative pressure level cleanroom is required depending on type of drug being compounded.
- USP797/800 cleanrooms typically require ISO7 compounding rooms, gloveboxes and ISO8 gown rooms.
- A modular cleanroom is an economical solution for the broad variety of USP797/800 cleanroom sizes.

CBD Extraction Cleanroom
A CBD Extraction Cleanroom is required for FDA regulated CGMP practise. Typical features include:
- ISO7 or ISO8 cleanroom with HEPA fan filter units to filter the air in the cleanroom
- Cleanable modular cleanroom walls
- Gowning room airlock to don cleanroom garments
- Magnehelic gauges to measure air pressure level of cleanroom vs. ambience.
FAQs Most Cleanroom Classifications
Q: What Is a Cleanroom?
A: A cleanroom (or clean room) is a room that has HEPA filtration to remove particles from the air. Cleanrooms are used for manufacturing where high levels of cleanliness and sterility are required. Mutual applications are medical devices, pharmaceutical and semiconductor manufacturing. The FDA mandates the utilize of cleanrooms to create GMP manufacturing factories.
Q: What Are Cleanrooms Used For?
A: Cleanrooms are used for manufacturing where loftier levels of cleanliness and sterility are required. Common applications are medical devices, pharmaceutical and semiconductor manufacturing. Cleanrooms use HEPA filters to remove particles from the air.
Q: How Clean Is a Cleanroom?
A: Very make clean. A class 100 cleanroom has 100 particles per cubic pes. By comparing your typical function space has between 500,000 and 1 one thousand thousand particles per cubic human foot. Cleanrooms come in dissimilar classes from class 100 to 100,000.
Q: When Is a Cleanroom Required?
A: Medical device and pharmaceutical manufacturing requires sterile environments to produce their products. Cleanrooms provide this sterile super clean manufacturing infinite which reduces the chance of contamination getting in your medicine. Semiconductor manufacturers produce devices with ultra small super dense features. Examples are computer chips for your cell telephone or PC. If contagion were to get on the chip during manufacturing, they would not work.
Q: What Does ISO Stand For?
A: ISO is the International Standards Organisation. It has created the ISO 4644-1 Cleanroom Standards that describe the allowed number of particles, the allowed size of particles and HEPA filtered air catamenia changes per hr meet ISO-4, ISO-five, ISO-half dozen, ISO-7, and ISO-eight standard. Information technology relies on measurements per cubic meter. It corresponds to the USA based Fed Standard 209E which relies on measurements per cubic foot. Fed Standard 209E corresponding classes are x, 100, m, 10k and 100k.
Q: What is Clean Room in Pharma?
A: In pharma a clean room is a controlled environment using HEPA filtration to minimize particulate contamination. Pharmaceutical manufacturers are subject to FDA validation of their manufacturing which typically specify use of a clean room to ensure the quality of the manufactured pharmaceutical product. Sterility is highest priority. Pharma cleanrooms focus on both not feasible (inanimate) and viable (live) contamination. They typically use laser particle counters to measure non viable contamination levels and settling plates with civilization media to mensurate viable contagion levels. Pharmaceutical cleanrooms use aggressive chemical and UV light cleaning techniques to maintain sterility.
Q: What Are Cleanrooms Used For?
A: Cleanrooms are used in any industry that wants to control contamination in their facility. It is common to see pharmaceutical cleanrooms, medical device cleanrooms, semiconductor cleanrooms, electronic cleanrooms, aerospace cleanrooms, food cleanrooms, USP797 compounding pharmacy cleanrooms and biotech cleanrooms. Cleanrooms are too used by the regime such as national labs, defense industries, and R&D labs at universities.
Q: What is a Information Cleanroom?
A: A data cleanroom is a secure isolation virtual platform that typically stores anonymized marketing information from multiple sources. It is used to protect privacy and share data from multiple sources. It is very different from physical cleanrooms used for manufacturing.
Q: How Do Cleanrooms Work?
A: Cleanrooms rely on HEPA or ULPA filtration to remove particles from the air and create an ultra clean surroundings. With sufficient air changes per hr and laminar air flow information technology is possible to reduce particulate count from greater so 500k/ft3in typical office space to as low every bit 100/ft3(form 100 cleanroom). Airlocks are used to prevent contamination from entering the cleanroom. Workers within cleanrooms typically wear cleanroom garments such every bit booties and bunny suits to forbid them from bringing contamination into the room. Eating and drinking are never allowed in cleanrooms.
Q: Who Needs a Cleanroom?
A: Industries such every bit pharmaceutical, medical device and USP797 compounding pharmacies are required past the government to manufacture in sterile surround and must utilize cleanrooms. Other industries such equally semiconductor, electronics, aerospace and optics find the ultra-clean environments in cleanrooms are the only way to toll effectively manufacture their products. Other industries that use cleanrooms include food, beverage, e-liquid, CBD and vitamins.
Q: What is a Pharmaceutical Cleanroom?
A: Pharmaceutical manufacturers are discipline to FDA validation of their manufacturing which typically specify use of a clean room to ensure the quality of the manufactured pharmaceutical product. Sterility is highest priority. Pharmaceutical cleanrooms focus on both not feasible (inanimate) and viable (live) contamination. They typically utilise laser particle counters to measure non viable contamination levels and settling plates with culture media to mensurate viable contamination levels. Pharmaceutical cleanrooms use aggressive chemic and UV low-cal cleaning techniques to maintain sterility.
Q: What is a Class 1 Cleanroom?
A: A class 1 cleanroom refers to ISO standard allowing less than two particles greater than 0.3 microns and no particles greater than ane.0 microns per cubic meter. A grade 1 cleanroom typically has from 500-750 air changes per hour and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors. It is the cleanest classification.
Q: What is a Course 2 Cleanroom?
A: A form two cleanroom refers to ISO standard allowing less than eleven particles greater than 0.iii microns and no particles greater than 1.0 microns per cubic meter. A course 2 cleanroom typically has 500-750 air changes per hour and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors. It is the 2nd most clean classification.
Q: How Many Types of Cleanrooms Are At that place?
A: The most mutual type cleanrooms are modular cleanrooms, stick-congenital cleanrooms (or standard), and softwall cleanrooms. Modular cleanrooms utilize prefabricated modular wall systems which let for faster installation, easy modification(s) and reasonable cost. Stick built or standard cleanrooms rely on conventional steel stud(s) drywall structure. It can be slightly cheaper. Softwall cleanrooms employ clear vinyl curtains suspended from metal frames. Small softwall cleanrooms are often mounted on casters and so they can exist hands moved around.
Q: What is a Class iv Cleanroom?
A: A grade 4 cleanroom refers to ISO standard allowing less than 1020 particles greater than 0.iii microns and less than 2 particles greater than five.0 microns per cubic meter. A course v cleanroom requires from 500-600 air changes per hour and typically utilizes ULPA filtration. Other mutual characteristics are 100% ULPA ceiling coverage and raised floors. It is the quaternary most make clean nomenclature.
Q: How Do You Set up For Cleanroon / Installations?
A: If you need an ISO-seven cleanroom you should fix your facility for the modular cleanroom installation. Bank check that area is complimentary and clear up to top of cleanroom. Often existing electrical conduit, lighting, sprinklers, and HVAC ducting need to be relocated. When installing new cleanroom floor brand sure the existing concrete is in skillful shape. Have any cracks and depressions filled to level the floor.
What Are the Do and Don'ts in a Cleanroom?
Do:
- Do wipe downwardly all surfaces on a regular basis to remove contamination.
- Exercise make sure doors are always closed to maintain positive pressure.
- Practise always take all staff were cleanroom suits over their street clothes to forbid bringing contamination into the cleanroom.
Don't:
- Don't eat or drinkable in your cleanroom.
- Don't bring muddy equipment or material into cleanroom – always wipe it downwardly earlier bringing information technology in.
- Don't turn off the HEPA fan filter units – information technology will take several hours later you turn them dorsum on before the cleanroom is clean over again.
ISO Cleanroom Specifications
The ISO ane specification for cleanrooms require less than 2 particles greater than 0.3 microns and no particles greater than 1.0 microns per cubic meter. An ISO 1 cleanroom typically has from 500-750 air changes per 60 minutes and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors.It is the virtually clean of the cleanroom classification.
The ISO 2 specification for cleanrooms requires less than 11 particles greater than 0.3 microns and no particles greater than i.0 microns per cubic meter. A IS0 2 cleanroom typically has 500-750 air changes per hr and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors.Information technology is the 2ndmost clean nomenclature.
The ISO 3 specification for cleanrooms requires less than 102 particles greater than 0.3 microns and no more viii particles greater than 1.0 microns per cubic meter. A IS0 three cleanroom typically has 500-750 air changes per hour and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors.It is the 3rd virtually clean nomenclature.
The ISO iv specification for cleanrooms requires less than 1020 particles greater than 0.3 micronsand no more than 2 particles greater than 5.0 microns per cubic meter. An IS0 4 cleanroom typically has 500-600 air changes per hour and typically utilizes ULPA filtration. Other common characteristics are 100% ULPA ceiling coverage and raised floors.It is the quaternary most make clean classification.
The ISO five is a super clean cleanroom nomenclature. A cleanroom must have less than 3,520 particles >0.5 micron per cubic meter and 250-300 HEPA filtered air changes per hour. The equivalent FED standard is class 100 or 100 particles per cubic pes. Mutual applications are semiconductor manufacturing and pharmaceutical filling rooms.
The ISO half dozen is a very clean cleanroom classification. A cleanroom must have less than 35,200 particles >0.v micron per cubic meter and 180 HEPA filtered air changes per hr. The equivalent FED standard is grade k or 1000 particles per cubic foot.
The ISO 7 is a common make clean cleanroom classification. A cleanroom must accept less than 352,000 particles >0.v micron per cubic meter and 60HEPA filtered air changes per hour. The equivalent FED standard is class ten.000 or 10,000 particles per cubic foot. Mutual applications are chemist's USP800 compounding rooms, electronics manufacturing and medical device manufacturing.
The ISO 8 is the to the lowest degree make clean cleanroom classification. A cleanroom must have less than 35, 200,000 particles >0.5 micron per cubic meter and 20 HEPA filtered air changes per hour. By comparing a typical office space would be 5-ten times more dirty. The equivalent FED standard is course 100,000 or 100,000 particles per cubic pes. Common applications include plastic extrusion for medical devices, e-liquid manufacturing, and nutraceutical packaging.
Source: https://www.americancleanrooms.com/cleanroom-classifications/
Posted by: millerbeftelf1970.blogspot.com


0 Response to "Is Rapid Clear Clean Pee Lab Grade"
Post a Comment